Modern Monoclonal Antibody Manufacturing: An Introduction
Monoclonal antibody (mAb) therapies continue to provide both powerful weapons against disease and compelling business opportunities for forward-thinking companies.
By Matthew Kennedy, Process Engineer
Now, forty years after the first monoclonal antibody therapy emerged, new indications and therapy types such as antibody drug conjugates (ADCs), bispecific antibodies, and multispecific antibodies are revolutionizing treatment options. New process and facility designs are redefining what it means to move fast and deliver these treatments to the patients who need them.
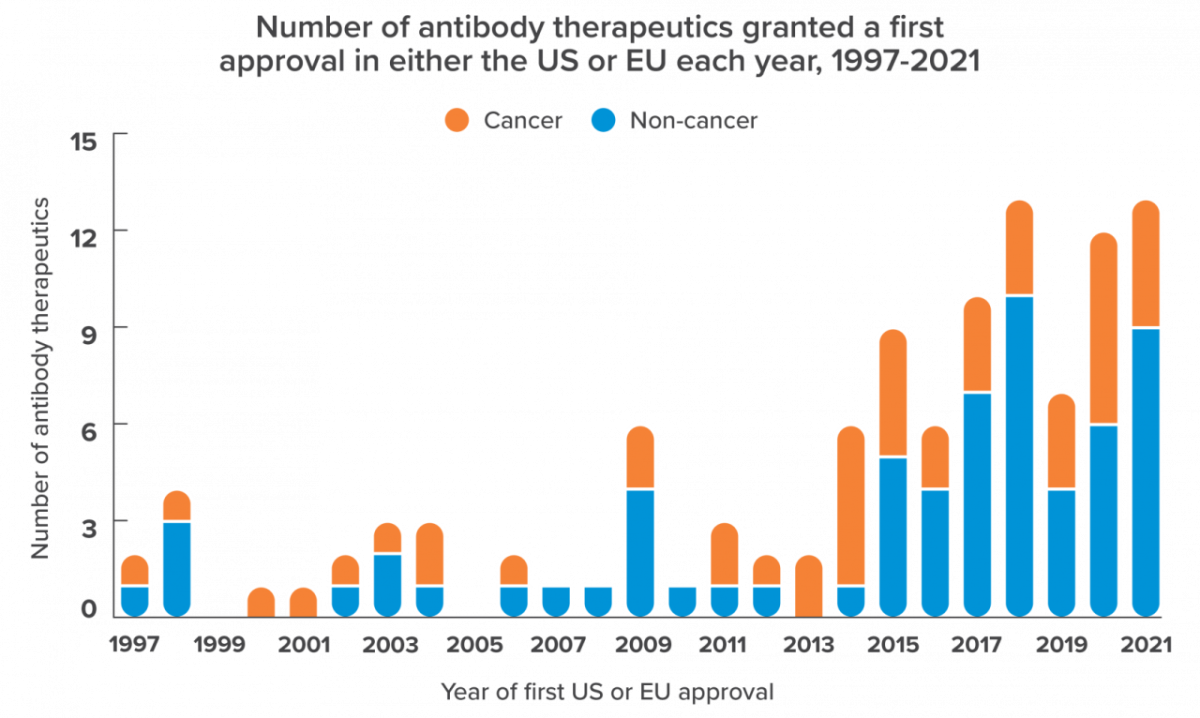
In 1986, patients suffering the effects of a rejected transplant found hope from a novel source: the FDA had approved the world’s first monoclonal antibody, Muromonab-CD3, an immunosuppressant indicated for organ rejection in humans. In 2021, the FDA approved the 100th antibody therapy on the market.
During the 35 years separating those milestone events, monoclonal antibodies were established as the majority contributor to the global biologics market. In fact, they represented about 70% of total biopharma sales in 2019—a 50% increase in just four years. Meanwhile, as approvals of antibody therapies accelerate and new combination therapies continue to emerge, one thing is certain: monoclonal antibodies are here to stay.
Today’s innovators and established manufacturers are moving the mAb industry toward an all-new frontier, where high-throughput screening and AI-powered protein design will de-risk the developmental pipeline and increase the overall value proposition of mAb products.
For startups, mature pharma companies, and contract development and manufacturing organizations (CDMOs) alike, the key to riding this wave of innovation into an exciting and sustainable future lies in asking the right questions early, and in planning your future manufacturing network strategy around the answers.
Questions for building a strong monoclonal antibody strategy:
- At what point in the development timeline should you invest in manufacturing capacity?
- What are the risks and advantages of flexible, highly scalable single-use technologies versus fixed stainless steel installations?
- How can you better standardize and intensify your process while staying flexible enough to rapidly adapt as your development pipeline evolves?
- How can you efficiently produce a variety of therapy types in your pipeline?
This article will explore the latest insights related to these and other important questions. Although we’ll focus on monoclonal antibodies in particular, the approaches and ideas explored below apply directly to other types of molecules including bispecific antibodies, multispecific antibodies, antibody fragments (fAbs), and some therapeutic proteins.
First, the basics: how do monoclonal antibodies work?
What are monoclonal antibodies and how do they work? Monoclonal antibodies, sometimes referred to as mAbs, are lab-made antibodies that are cloned from a single parent cell (hence their prefix, ‘mono-’), and they’re engineered to bind to a single foreign antigen.
The human body typically takes several weeks to naturally manufacture a collection of antibodies in response to a specific antigen. Monoclonal antibody manufacturers have learned to recreate and accelerate that process in the lab, effectively giving patients’ immune systems an enhanced weapon against many diseases, including oncological and non-oncological indications.
Beyond monoclonal antibodies are bispecific antibodies (BsAbs) and multispecific antibodies. These proteins are made up of two (‘bi-’) or more (‘multi-’) monoclonal antibodies, making them capable of recognizing and binding to two or more different antigens. This capability enhances their potential immune function and makes them an especially versatile immunotherapeutic tool.
Terms to know
Antibody drug conjugates (ADCs)
Therapeutic proteins
Drug substance
Drug product
Titer
Process intensification
Manufacturing network strategy
Multimodal manufacturing
What are the benefits of monoclonal antibodies?
mAbs are versatile.
Monoclonal antibodies are the Swiss army knife of the biopharmaceutical market. They have proven benefits as targeting molecules, analytical tools, components in a combination therapy, or as stand-alone therapies.
mAbs are capable of high specificity.
Monoclonal antibodies can identify cancers or deliver anti-cancer drugs to target cells with greater efficacy and fewer side effects than other oncological treatments.
Their applications aren’t limited to oncological treatments, however. Monoclonal antibodies also play a key role in fighting asthma, auto-immune disorders, and some viral infections, including COVID-19. It may even be possible to use them as a prophylactic vaccine. Meanwhile, they’re helping to define the future of biotech; for example, innovative researchers are conscripting mAbs as vehicles to target the delivery of novel RNA gene therapies to specific cells in the human body.
Advances in drug discovery have shortened the developmental pipeline.
The sooner researchers can eliminate subpar molecules from their R&D pipeline, the faster they can get successful molecules to market. Rational protein design and high-throughput screening are helping mAb manufacturers meet this challenge by giving them the tools they need to find new antibodies and match them to targeted disease conditions more rapidly and accurately.
These manufacturers may soon get another major boost from emerging technologies like AlphaFold, which leverages artificial intelligence to predict a protein’s therapeutic potential, potentially shaving months or even years from the development timeline.
Additionally, advances in cell line development such as targeted gene integration, single cell seeding, and medium optimization significantly reduce the time required to develop a high-productivity cell line that expresses high-quality protein.
Established manufacturing methods help companies scale to commercial production.
In contrast to all-new areas of and specially engineered therapies, the process of scaling mAb production from the R&D lab to the commercial production facility is well-defined, both in terms of the platformed technologies involved and the regulatory pathway. Meanwhile, technologies that support robust strategies such as closed processing are rapidly evolving, reducing the risk of contamination and product loss while further streamlining the pathway to commercial manufacturing.
How are monoclonal antibodies produced?
Monoclonal antibody manufacturing generally involves culturing a genetically modified organism to express the target protein, followed by downstream chromatography and filtration steps.
Although this process is relatively standard across the industry, a key difference from one manufacturer to the next has to do with the technologies behind their manufacturing approach.
Stainless steel technologies are the traditional choice for large-scale manufacturing, while single-use technologies have gained popularity because of their agility. For some manufacturers, a hybrid manufacturing technology approach designed to amplify the benefits of both stainless steel and single-use technologies (while minimizing their drawbacks) is the best way forward.
Flexible fixed stainless steel manufacturing technology for mAbs
When designed around a range of potential processes, a facility featuring fixed technologies can support a number of different products while holding its value, even as product pipelines or products change.
Benefits of stainless steel systems:
- Volume
- Supports high-volume throughput, which may be particularly necessary for low-titer processes.
- Suitable for approved molecules or biosimilars with a large patient population/broad indications.
- Supply chain
- Offers potential for a lower cost of goods.
- Protects against the full impact of external supply chain risks related to quality and availability of single-use components.
- Testing and validation
- Stainless steel has been rigorously tested in terms of leachable and extractable risks.
Challenges of stainless steel systems:
- Capital investment and project delivery
- Requires a significant upfront, at-risk investment.
- Raises the financial risk for project owners (if it is narrowly designed).
- Requires early capital investment in design and construction, despite uncertainty of regulatory approval.
- Might necessitate a costly shutdown or extensive renovation if alterations are required.
- Production schedule
- Requires clean-in-place and sterilization-in-place and associated validation, which impacts manufacturing efficiency.
Single-use manufacturing technology for mAbs
A reputation for supporting fast and flexible processes has popularized single-use technologies among antibody manufacturers, particularly those working with lower volumes.
Benefits of single-use systems:
- Capital investment and project delivery
- Increases speed to market and shortens construction timelines.
- Permits deferral of capital investment in manufacturing capacity until there is greater certainty in regulatory approval.
- Flexibility
- Modular design and platform processes readily enable flexible facilities.
- Enables manufacturers to scale-out rapidly in response to shifts in market demand forecasts.
- Speed
- Supports a faster product changeover, potentially increasing throughput.
- Helps accelerate candidates through early clinical trials, process development, and launch.
Challenges of single-use systems:
- Environmental impact
- Generates a large volume of waste, from bioreactors to hold bags.
- Contributes to overall carbon footprint through the manufacture, distribution, and disposal of single-use components.
- Supply chain
- Requires a robust sourcing strategy to ensure a reliable supply of components.
- Also requires a strategy to assess supplier suitability and ensure connectivity between proprietary components.
- Footprint
- Requires a facility large enough to accommodate scale-out.
Hybrid stainless steel and single-use strategy for mAbs
The choice between a fixed technology system and a single-use strategy is not necessarily binary. Many manufacturers generate value by combining these approaches in a hybrid system that optimizes cost, robustness, and speed to market.
Who is manufacturing monoclonal antibodies?
The monoclonal antibody industry is a meeting ground for mature pharma companies seeking to diversify their drug product portfolio, research labs with promising molecules in development, and CDMOs with the capability to support some of this production capacity.
To succeed in such a crowded marketplace, each of these manufacturers needs a strategy that’s right-sized for their unique business case, drug product portfolio, process requirements, and other needs.
Mature pharma companies
Key focus: Modernizing certain existing assets quickly and cost-effectively while adding additional flexible manufacturing capacity.
Mature companies may have the capital necessary to acquire intellectual property that was developed externally, empowering them to balance their R&D pipeline with both antibodies and other therapies.
However, integrating those externally developed molecules could prove challenging, and if they have fixed, product-dedicated facilities in their manufacturing network, they may find it difficult to scale their pipeline efficiently. This raises several important questions:
- Without knowing which candidates will succeed and when, how do we design and implement a robust commercialization strategy? How can we balance the need to support many different low-volume products today with the possibility that we may need larger volumes in the future?
- Where should we focus our capital spending: on rebuilding our depreciating assets, or on designing and constructing all-new facilities?
- Should we invest in purpose-built capacity, or should we design for flexibility from day one?
Biotech startups
Key focus: Preparing for rapid commercial launch while mitigating capital investment risk as molecules move closer to approval.
Startups need to carefully manage their burn rate as they proceed through successive rounds of investment, demonstrating success along the road to commercialization. Betting too early on manufacturing capacity could have substantial financial consequences; on the other hand, deferring capital investment too long could mean missing out on market demand during the crucial exclusivity period. To overcome these challenges, biotech startups should ask themselves:
- Are there benefits to building our own facility, or
- If we decide to build our own facility, how do we know when the time is right to commit?
- How do we ensure that our facility can adapt to process changes that might arise during development?
- How can we future-proof our facility so that it remains an attractive asset as manufacturing technologies change?
- How do we design our facility so that it can rapidly pivot in the event that our candidate molecule does not make it to market?
CDMOs
Key focus: expanding rapidly and strategically in order to meet today’s enormous demand for contract manufacturing capacity.
It’s a good time to be a CDMO with expertise in mAb manufacturing. A lack of available capacity and unprecedented capital investment in the biopharma industry have created a lucrative situation in which demand outstrips capacity. CDMOs who are able to invest in expanding their capacity are all but certain to find clients. However, along with these advantages comes a set of challenging questions:
- How can we accelerate our capital project delivery timeline to take advantage of today’s demand?
- How do we design a standard infrastructure that will allow us to campaign different products or multiple modalities through our facilities, maximizing our throughput and minimizing costly downtime while maintaining regulatory compliance?
- How can we design flexible systems and facilities capable of supporting multiple clients with unique needs and product types, such as different purification platform needs or the need for multimodal flexibility?
- How do we standardize our facility design and operation approaches to improve efficiencies across our manufacturing network?
In the second half of this article, we’ll look into what goes into designing that forward-thinking plan, from choosing the right manufacturing technology approach to overcoming the challenges of a fast-moving and competitive marketplace.
What are the challenges of producing monoclonal antibodies?
Manufacturing challenge #1:
Manufacturers are under pressure to increase speed to market.
Like building an airplane while in the air, mAb manufacturers have to juggle many complex factors as they race their competitors to the marketplace: regulatory approval, market demand, competitive positioning, available manufacturing capacity–the list goes on.
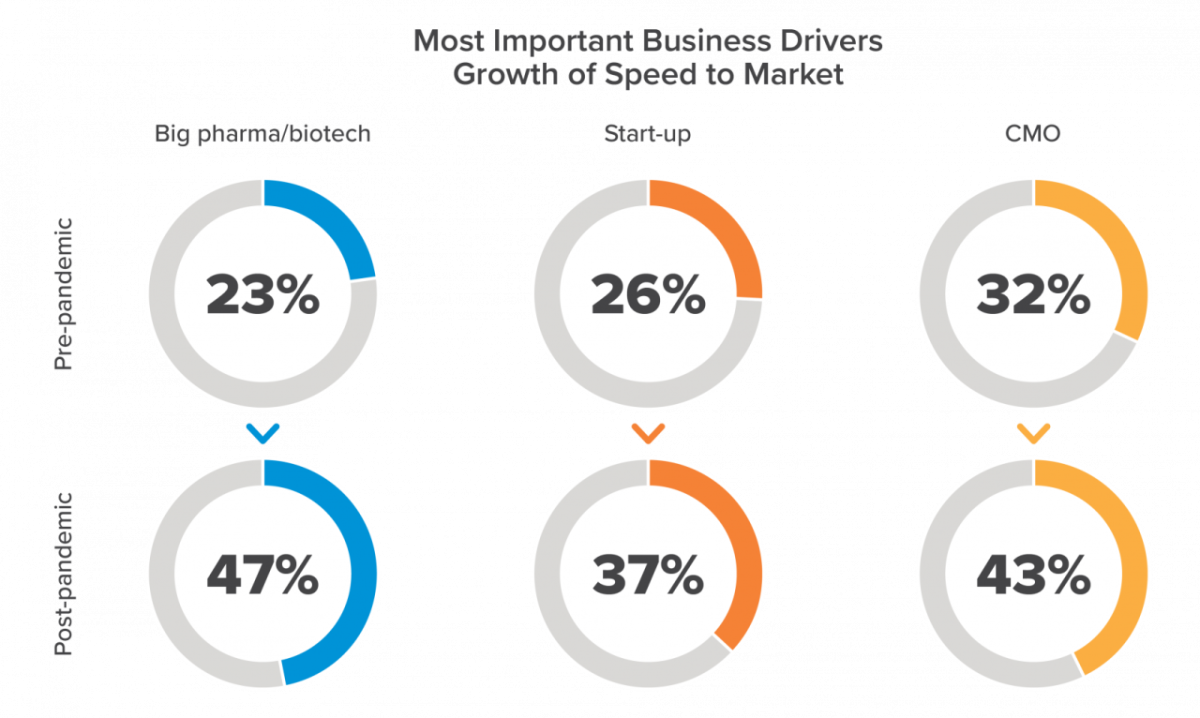
Success depends on getting each of those factors right, and on doing it all under tight scheduling constraints. That’s especially true in the wake of the COVID-19 pandemic, which introduced expedited regulatory pathways and redefined what it means to move fast in the life sciences industry. In fact, speed to market has significantly increased as a business driver across all manufacturing types, according to research from the 2021 Horizons: Life Sciences report which leverages survey responses of more than 500 industry leaders.
One of the more reliable and important ways to increase speed to market while readying for future growth is by prioritizing flexibility in multiple ways:
- Strategic facility planning: Do you need multi-product launch facilities designed for rapid scale-out or a larger-scale facility optimized for higher throughput–or some combination of the two? How will you leverage strategic partnerships? Strategic facility planning will uncover the answer to these questions, ensuring that your business objectives align with your overall manufacturing network strategy.
- Integrated project delivery (IPD): This approach, an alternative to traditional design-bid-build project delivery, relies on collaboration, respect for people, and shared accountability to deliver projects faster and with greater flexibility. At CRB, we call this integrated approach ONEsolution.
- Modular manufacturing: By designing for rapid repurposability at the facility level, manufacturers can campaign between products and support multiple processes with minimal downtime.
Manufacturing challenge #2:
Platform manufacturing can improve speed and lower costs, but manufacturers don’t always have time to standardize from the start.
Under pressure to move fast, R&D teams may feel the need to quickly develop a process that will work for a single product of interest. This approach might save time in the lab, but it will cause major bottlenecks in downstream production when manufacturers have to develop bespoke capabilities for each successful product in their pipeline.
Standardization is the solution. The key is to think in terms of ranges, not point values. These ranges become guardrails for R&D teams, who can then align their process development efforts with the capabilities of their company’s existing or planned commercial manufacturing network. Unless driven by a compelling financial or quality-related reason, deviations are discouraged. This sets up manufacturers to mix and match processes and facilities according to the needs of a multi-product portfolio while maximizing the economies of common cell lines, media formulations, resins, buffer systems, analytical tests, and other resources.
When planned well and established early, this standardized approach lays the groundwork for a fast-moving, high-throughput mAb development pipeline, which in turn feeds a manufacturing operation that’s capable of rapidly integrating new products. This approach also allows an early and comprehensive cost of goods analysis to improve the competitiveness of therapies in the market.
The trouble is that not every team has the bandwidth or resources to plan for standardization from the start. That’s where an expert partner can help. With the right know-how and experience working for them, mAb manufacturers who missed out on early opportunities to standardize their process can still develop an effective commercial manufacturing approach. The key is in expertly assessing the many options available—from bespoke solutions to platformed, off-the-shelf equipment technologies—and designing a facility that can ultimately accommodate any number of non-standard processes as efficiently and cost-effectively as possible.
Manufacturing challenge #3:
Manufacturers must balance the intensification process.
Over the last fifteen years, cell line engineering and medium development have enabled monoclonal antibody manufacturers to more than double their average upstream titers—that is, the amount of protein expressed relative to bioreactor volume.
High-titer processes offer many advantages in upstream manufacturing. They help squeeze more productivity from your bioreactors, which means you can use smaller, single-use equipment and less space to achieve the same throughput as a lower-titer process. Higher titers can also improve your flexibility, making it possible to rapidly campaign between products or to adopt a different process with minimal renovation.
Manufacturers can also intensify their upstream process through bioreactor pooling, N-1 perfusion, N perfusion, standard fed-batch or concentrated fed-batch production. Some manufacturers might further intensify their upstream process by relying on multiple seed labs or concentrated cell banks to increase campaign cadence and reduce scale-up time. These approaches can help to intensify production in existing facilities or improve the commercial viability of a low-titer process.
Process intensification isn’t a magic bullet, though. The more productive your upstream process, the more pressure you’re shifting to your downstream and support operations. In-process hold volumes, unit operation cycles or sizing, cleaning and sanitizing operations, waste processing—these can all become rate-limiting without an expert plan in place.
A more productive upstream process also introduces the challenge of acquiring, paying for, and storing more media and buffer—a challenge which multiplies as your productivity grows. Buffer and media concentrates with in-line dilution or in-line conditioning may help relieve this pressure. Multi-column chromatography, which reduces buffer consumption by maximizing your resin capacity, as well as in-line concentration are other ways to preserve the process density you worked so hard to gain upstream. These approaches can further impact your utility systems and facility infrastructure.
The future of antibody manufacturing is all about flexibility
Since that first approval in 1986, mAb manufacturers have learned so much about what it takes to efficiently and consistently produce these life-changing molecules for a global market. But, on multiple levels, there are many more advances to come.
New approaches to discovery, such as emerging AI systems capable of predicting protein structures quickly and accurately, are paving the way for new products to emerge. At the same time, techniques such as targeted gene integration and medium optimization are accelerating the process of making highly productive, high-quality cell lines. Continuous technologies, designed for faster campaigning and a more compact facility footprint, are also emerging as a potentially viable strategy in the near future.
What does all of this mean for monoclonal antibody manufacturers? It means that success will depend on your ability to leverage existing knowledge while staying flexible enough to adapt as future technologies and processes cross the starting line, changing the antibody manufacturing landscape yet again.
Finding your partner
It takes expertise to maintain a high level of flexibility and ensure that you’re minimizing your risks while accelerating your speed to market. At CRB, we’ve developed that expertise over hundreds of projects, helping our clients align their manufacturing strategy to their projected product portfolio by integrating modular designs, hybrid process technologies, and scale-out approaches that offer the potential to support multiple therapy types. These techniques are the key to delivering complex product portfolios faster and more reliably.
If your pipeline includes monoclonal, bispecific, or multispecific antibodies and you’re ready to establish a commercialization strategy that fits your unique situation, let’s talk.