Partnering to Advance COVID-19 Treatment
Originally published on UnitedHealth Group website, December 4, 2020
Potential treatments continue to emerge in the fight against COVID-19. And for people at severe risk of the disease, new treatments may help accelerate recovery and reduce the need for hospitalization. However, real-world research is needed to test them in large and diverse populations.
As part of an effort to aggressively explore promising new treatments to intercept this disease, UnitedHealth Group is partnering with Eli Lilly to study its new monoclonal antibody treatment, bamlanivimab, for non-hospitalized people recently diagnosed with COVID-19. The effort will leverage UnitedHealth Group’s broad reach and expertise in health care, as well as its industry-leading technological prowess and commitment to ingenuity throughout the pandemic, in combination with Lilly’s expertise in pharmaceutical development.
The treatment — authorized by the U.S. Food & Drug Administration (FDA) for emergency use in people who have mild to moderate symptoms — may boost the human immune system to help more quickly eliminate the virus that causes the disease, and in initial research has been shown to reduce the risk of hospitalization when used in people who have early-stage COVID-19.
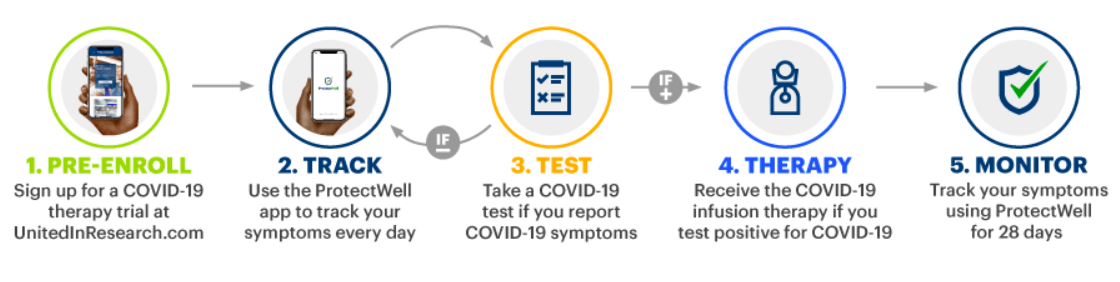
How it works
UnitedHealth Group will use an integrated combination of digital tools, real-world data, and on-demand clinical support to create a personalized and seamless observational study experience. UnitedHealthcare Medicare Advantage members in 46 states who meet the FDA’s criteria for the treatment will be invited to volunteer for the study through United in Research — UnitedHealth Group’s clinical research platform and virtual community.
Those who are approved for the study and are in an area where they can receive treatment will then be directed to download the ProtectWell app and begin tracking their symptoms. Should volunteers indicate through ProtectWell that they are experiencing symptoms, UnitedHealth Group will arrange for them to be sent or take a COVID-19 test. Individuals who test positive for COVID-19 will be contacted by an Optum infusion nurse to schedule a home infusion treatment of the therapy.
Study participants will be virtually monitored through daily app-based symptom-checks for 28 days after their infusion treatment. Any participants who report feeling worse will be reminded to seek medical care if they experience severe symptoms and will receive a telemedicine visit with a nurse. Based on the telemedicine visit, participants will be further advised to remain at home or seek medical care.
“This partnership with Lilly is a robust multi-faceted collaboration, grounded in helping people and improving health through innovations that better prevent, detect and intercept disease. We look forward to combining our expertise to advance a range of therapies that transform health, get upstream of other chronic diseases, and achieve the best care possible.”
– Ken Ehlert, Chief Scientific Officer, UnitedHealth Group and Chief Executive Officer, OptumLabs

